Choi CARE
Viral Transport for Virus, Chlamydia, Mycoplasma & Ureaplasma
Choi CARE Clinical Viral Tranport Medium (CTM) is intended for the collection and transport of clinical specimens containing
Virus, Chlamydia, Mycoplasma and Ureaplasma from the collection site.
The test precedures for quality control are based upon the quality control methods described in CLSI M40-A2, FDA (CDC) SOP #: DSP
-052-04 and others. CTM transport medium is stable for 24 months at room temperature. Specimens in CTM medium are stable for
48 hours at room temperature.
Aplication
- All Viruses (Influenza A & B, Herpes Simplex, I and II, Cytomegalovirus, Respirato
- Chlamydia tachomatis and penumoniae
- Mycoplasma hominis and penumoniiae
- Ureaplasma urealyticum
- Enzyme Immuno Assays (EIA)
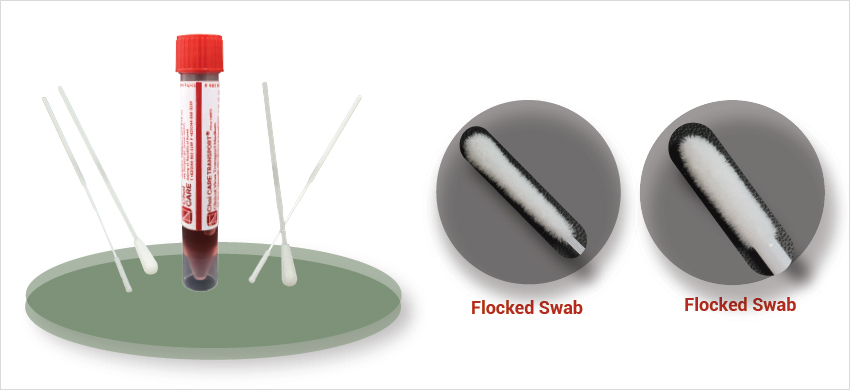
Choi CARE Clinical Viral Tranport Medium® CTM
- CTM medium transport specimens in safety by using self-standing conical
- There are various swab applicators for the sample collection
- Specimens in CTM are stable for 48 hours at room temperature





